The carbon isotope with mass 14, known as radiocarbon, is one of the unstable isotopes of carbon
with widespread applications in the scientific world. The use of 14C as a „clock” for
estimating the age of various historical and pre-historical samples is one of its most
important applications.
Willard F. Libby was the father of the radiocarbon dating method who mentioned the possibility
to date the carbon based samples for the first time in May 1947 within the “Science” journal
and then applied this method for the first time in 1949. For his scientific contribution
W.F. Libby was awarded with the Nobel Prize in Chemistry in 1960.
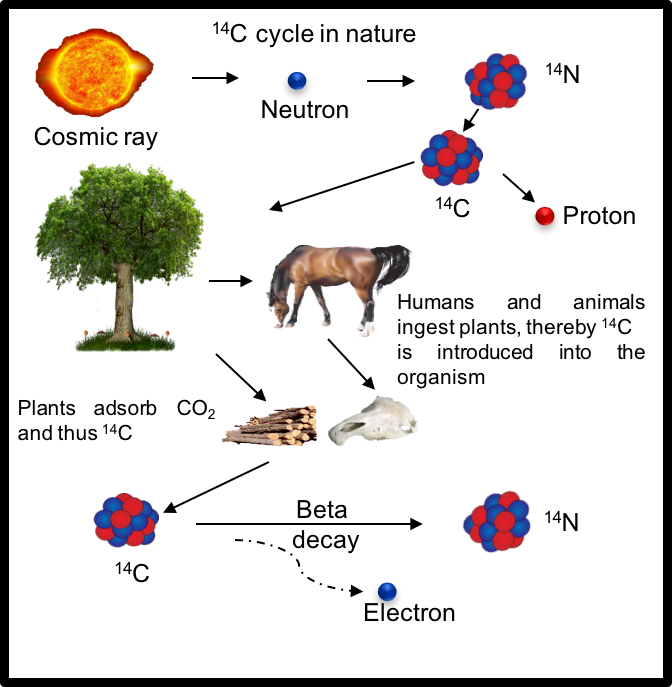
Small amounts of 14C are generated in the upper layers of the atmosphere under the influence
of cosmic rays, especially high energy protons, being produced as a result of the interaction
of radiation with the most abundant element of the atmosphere, 14N. The resulted radiocarbon
chemically reacts with oxygen to form 14CO2 which enters the global cycle of carbon in nature.
During their lifetime, plants and animals intake 14C from the carbon dioxide present in the
atmosphere, from water and nutrients, reaching an equilibrium level of 14C with the environment
(~1.2*10-12 from 12C). Once the organism stops living, the carbon exchange with the environment
stops as well, thus the concentration of radiocarbon from the organism decreases at a specific
rate described by the radioactive decay law with a known half-life T1/2 = 5730 years.
From 1949 to 1977 all radiocarbon dating analysis were made by radiometric measurements.
From 1977, the radiocarbon dating method that makes use of a particle accelerator, also
known as Accelerator Mass Spectrometry method, gained a lot of notoriety. Among the
advantages of this method we can name reduced analysis time, here including also
chemical preparation of the samples, the amounts of necessary dating material
(a few grams to milligrams) and high measurement accuracy.
The radiocarbon dating laboratory RoAMS from IFIN-HH applies the AMS dating technique
using a 1MV Tandetron Accelerator (produced by High Voltage Engineering Europe).
The radiocarbon dating method AMS implies counting atom by atom 12C, 13C and 14C
species from the sample in order to determine the isotopic ratios. Measurement
efficiency can reach 10 -15 (14C/12C) which makes AMS the most sensitive radiocarbon
dating method.